Pharmacy Inventory
Utilizing the Pharmacy Inventory System in ZIMS
Design Note on Pharmacy Inventory in ZIMS:
The main purpose of the Pharmacy Inventory module is to provide assistance with keeping records for government regulated drugs. In situations where a user is legally required to maintain records for specified drugs and would already be expending resources to track purchase, usage, loss and discards of these drugs, this module can partially automate the process and make it easier to maintain the required records.
The module will work with other non-regulated pharmaceutical drugs prescribed and dispensed in discrete doses – e.g., antibiotic tablets. However, institutional resources (time and effort) are required to enter non-regulated drug purchases into the inventory module and to maintain dispensing records for each drug. These additional costs provide few benefits for the institution and most users do not find it worthwhile to attempt to track usage of non-regulated drugs through the pharmacy inventory module.
The module is not designed to track drugs and other items that are not prescribed using quantified doses – e.g., topically applied ointments and sprays. Without accurate measures of usage, automating calculation of the remaining amount in a container, based on prescription or anesthesia records is not possible.
Finally, vaccines, vitamins, fluids and nutraceuticals are specifically excluded from this module. Users should not attempt to bend system rules by misclassifying these items within the treatment dictionary in order to allow usage within the pharmacy inventory module. An accurate global treatment dictionary is necessary to drive calculations of the Global Drug Usage Extracts and the Global Anesthesia Summaries resources, and Species360 staff will correct misclassified drug entries that we identify.
Utilizing the Pharmacy Inventory System in ZIMS:
Navigate to the pharmacy inventory via Start > Medical > Pharmacy Inventory menu options:
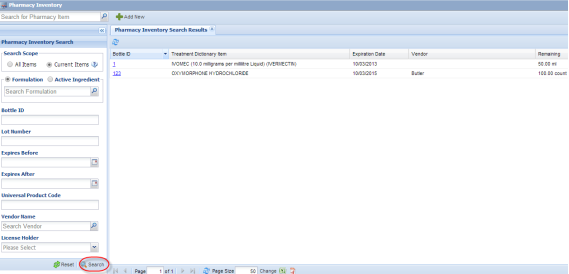
Upon opening Pharmacy Inventory module, you will be on the main search screen. The default scope is current pharmacy inventory items; leave the search parameters blank and click the Search button, to load all current items into the search results grid. Filter the list of inventory items using other fields in the search panel.
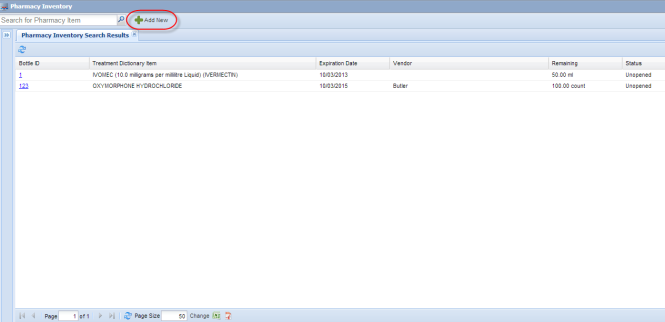
An item in the inventory is a single bottle or other container containing a specified starting amount of the drug (or drug combination) in the specified formulation. For example, a bottle of 5 mg diazepam tablets with a starting count of 10 tablets could be an inventory item. You must assign an individual and unique identifier to each container to allow tracking of dispensed amounts for that item. Module reports include a “bottle” report that summarizes the purchase information, usage from linked prescription and/or anesthesia records, and any loss or discards for that inventory item.
Regulated drug items are associated with legally required records and usage records for these inventory items are “locked” once an item is marked as “finished” within the inventory system. Locking item records prevents changing existing usage records or adding new usage records for that item, ensuring the integrity of your pharmacy inventory records. This locking ability and records that account for regulated drug usage on a bottle-by-bottle basis are key to meeting DEA record keeping requirements. If an item is accidentally locked before all usage records have been entered, the medical admin can unlock that item, but a record of the unlock process (and the reason) are maintained as part of the records for that inventory item.
Adding a New Inventory Item:
Click "Add New" button at the top of the Pharmacy Inventory screen to add a new item to your inventory
The “Add New Pharmacy Item” screen will appear, allowing addition of a new bottle or other container of a drug to the inventory.
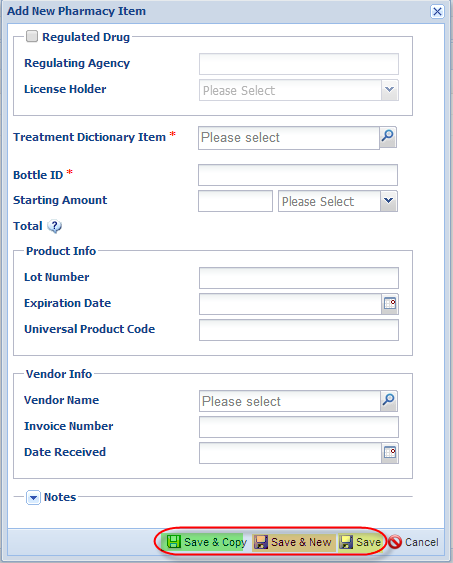
When you are entering a new pharmacy item, please note the following:
- Red asterisks denote the required data fields
- If the item is a regulated drug (you are legally required to keep inventory records), check the “regulated drug” box and then the regulating agency and license holder fields will become active. License holder drop down is populated from your Staff list and regulating agency from the Institution list in ZIMS
- Bottle ID (a drug container identifier) is a unique number that you assign to the drug bottle/container as it goes into inventory; that identifier should be written directly onto the container itself so that all dispensing activity can easily be linked to the correct inventory item. Prescription dispensing records and anesthesia records use this bottle id to track drug usage from this specific container. While you can make this bottle identifier as complex as you want, remember that you will have to enter this information into anesthesia and prescription records as you track drug usage; simpler, shorter identifiers will have an advantage during that data entry phase
- You have three options for saving data.
- If you are entering one item, just use the save in yellow.
- If you entering several different items, the save in orange will save your entry and wipe the contents so you can start uninterrupted entry on the new item.
- If you creating several entries of the same item (e.g., 6 bottles of ketamine just purchased from the same vendor), the save in green will save your current entry and keep the core information so you only have to enter the information that has changed. This will significantly reduce data entry efforts when items are purchased in bulk.
Bottle Information Details:
A search of the pharmacy inventory yields a list of items that match based on the selection criteria chosen. The left column of the results grid displays the Bottle ID for the item with a hyperlink.
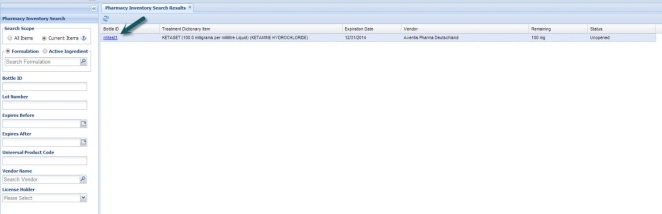
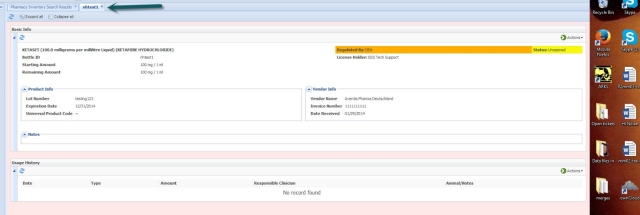
Clicking the Bottle ID Hyperlink opens a new tab with complete details for that item, including the purchasing information, current item status and any existing prescription or anesthesia usage records for this item.
Best Practices for the Pharmacy Inventory:
The following topics should help you better understand the appropriate use of the Pharmacy Inventory module.
Vaccines and Nutraceuticals...
Not supported by this version of the pharmacy inventory module. Do not attempt to bend the rules to enter these types of items into the inventory as routine maintenance the drug dictionary is required for the Drug Usage Extracts and Anesthesia Summaries resources and that maintenance will disable tracking of these types of items within your inventory.
Ointments, sprays and other topical drug treatments
…
It is not possible to automate usage tracking for pharmacy inventory items that are not used in discrete and easily quantified amounts. Attempts to use this module to track the usage from jars of ointment or spray bottles of flea medication will not yield satisfactory results; we strongly recommend not adding these types of items to the pharmacy inventory.
Tracking a bottle start to finish...
When tracking regulated (controlled) drugs, there are a few tips that will ensure your records are accurate and complete.
Registration:
- Add items using the specific tradename and formulation information. This will produce more flexibility during data entry of prescription and anesthesia records. If the drug container has been registered as Ketaset (100 mg/ml solution) (Ketamine), then anesthesia records that use either Ketaset or ketamine as the drug entry will be able to link to the inventory record. However, if a bottle registered as the generic product Ketamine (100 mg/ml solution) (Ketamine) will not link to the inventory records if the user (looking at the bottle label) enters Ketaset into the anesthesia record (there is no Ketaset registered in the inventory).
- Log/register each bottle in ZIMS as it arrives at your institution and write the unique Bottle ID onto the item as it is registered. You probably have easiest access to the DEA required vendor and invoice information, as the drugs are unpacked. This workflow also ensures that every item in the secure drug cabinet is already in the ZIMS system; you cannot end up in the field doing an immobilization with an unregistered bottle of ketamine that has no bottle id and will require extra work to fix all the worksheets and the records when the procedure is completed.
- We recommend you enter the starting amount in milligrams and record all usage in milligrams. Volumes can be more ambiguous; calculating the amount used depends on the concentration of the drug and if there is an error in the concentration, it impacts every usage record that is linked to a volume of drug
- Do not forget about the “save and copy” feature during inventory registration. If you have just received 10 bottles of the same drug, most of the information entered will be the same for each bottle. Using the Save and Copy, ensures that information such as drug name, vendor, invoice number, lot number and expiration date are retained for the next entry, and often just the unique bottle id will need to be entered for each additional item.
Marking as Finished/Closing an item:
- When a bottle of drug is physically empty, the clinician responsible for the bottle (usually the license holder) should go to the details tab for the item and review all usage records to ensure that the ZIMS records account for the entire contents of the item. If the records are complete and correct, the item can be marked as closed (Actions menu, top right, of the item details screen). Marking the item as finished locks the existing usage records (no edits allowed) and prevents anyone from creating a new usage record for that item.
- Once a bottle is marked finished, we recommend printing the final bottle usage report, then sign and date the report and file with your other DEA records to make it easier to show compliance during an audit of your drug inventory.
Note: For regulated drugs, if there is a discrepancy greater than 10% between the starting volume and the usage records, you will not be able to close the item. This amount of error between physical usage (the bottle is empty) and the recorded usage is not considered acceptable to meet DEA requirements. Review and correct usage records as needed and when the recorded usage is within 10% of the starting amount, mark that item as finished.
Modifying a closed bottle:
- Mistakes happen and you may end up with an inventory item that is marked as finished, but some usage records need modification. Only a medical administrator at your institution can reopen a bottle marked as finished. ZIMS will automatically create an audit trail, noting the date and responsible party that reopened the item. This audit trail information is in red text at the bottom of the bottle usage details screen, and cannot be edited or removed. Records for your controlled/regulated drug inventory are a legal obligation. Revisions made after the bottle has been marked finished should be uncommon and justifiable to regulating authorities. Careful review of records prior to marking a bottle as closed should minimize the need to re-open them for revisions.
Special circumstances:
-
Bottle records without formulation information
: If you had bottles migrate into ZIMS from MedARKS, you may have some inventory registered with the starting amount value in only mg or only ml and no drug concentration or formulation information. This can cause problems when the system attempts to calculate remaining amounts for the item.
It is important in these circumstances to be consistent with your data entry and create usage records that match the unit of measure used when the bottle was registered .
Examples:
- If a bottle of tramadol was registered as containing 100 tablets with no concentration listed, the prescriptions need to be formulated as follows:
Select “tramadol” for drug name. Write your prescription to include a dose (50mg) and dosage as usual. Add the concentration in the box on the lower right (50 mg tablet) and ZIMS will calculate that the amount administered for each dose is “1 count”. Dispensing/usage records should indicate the number of tablets given out. This ensures that your inventory will calculate properly.
- If a bottle of tramadol was registered as containing 5000mg without a tablet count, you would need to formulate the prescription as follows:
Select “tramadol” for drug name. Write prescription to include a dose (50mg) and dosage as usual. Do NOT add the concentration in the box on the lower right. ZIMS will indicate “50mg” as the amount administered for each dose. Dispensing/usage records should indicate the total number of mg used. All usage records for this bottle will be in mg, ensuring your inventory records calculate properly.
Note: For bottles registered with only mg or only volume starting amounts and no drug concentration/formulation information, but with mixed usage records (some usage in mg and some in ml), the system will not be able to correctly calculate remaining amounts. If you see this discrepancy in your inventory records, you will need to go into each usage record and edit it to match the unit of measure that designated when the bottle was registered. This editing process can be confusing; contact support@species360.org if you are having problems with these historical records. To avoid this problem with new inventory records, consistently register new bottles using the best practice recommendations detailed above. You will gain flexibility within the prescribing process, and your inventory records will remain accurate.
Drug Mixtures/”Drug Cocktails”:
- Commercially purchased mixtures of drugs are not a problem for the pharmacy inventory. The manufacturer specifies the mixture of drugs and the amounts of each drug within a single bottle or vial. Purchase, registration and usage from that single drug container is handled the same as any other pharmacy inventory item, with each entry recording the total volume or amount (mg) of drugs used from the container.
However, when you mix two controlled drugs from separate bottles into a single dart or into a single bottle to produce a custom formulation, you remain required by law to report the usage from each individual drug bottle. Under these conditions, rather than creating a new drug dictionary entry called BKX that states the concentration of butorphanol, ketamine and xylazine in your custom mix and then registering a bottle of that mixture in your inventory, you will generally find it easier to maintain separate inventory items for each purchased bottle or vial.
Example: You purchase a bottle of Ketamine and a bottle of Telazol (tiletamine & zolezepam) and assign them bottle id’s of K1 and T1 respectively. Now take 5 ml of liquid drug from K1 and add it to the 500 mg T1 bottle to dissolve that drug (supplied as a powder). The T1 vial now contains a custom (non-commercial) Telazol-ketamine mix in the same vial. Each ml of your TK mixture has about 100 mg of Ketamine and 100 mg of Telazol. You could create this TK mix in the drug dictionary as a compounded item, relabel the T1 vial as TK1, create a new pharmacy inventory item and record all usage against the TK1 bottle id. However, both of these drugs are specified as controlled substances by DEA and legally you need to be able to completely report usage from each purchased bottle of each drug. Specifying usage for a TK1 item in the inventory, makes reporting usage for the purchased bottles K1 and T1 becomes much more complicated.
For this reason, we recommend that you track usage from each original bottle separately within the ZIMS records. Label the T1 vial to make it clear that it now contains 100 mg/ml ketamine from bottle K1 in addition to the Telazol. Whenever the drug mixture is used, you will actually make two ZIMS entries – one entry saying xx mg was used from bottle T1 and the second entry saying yy mg was used from bottle K1. ZIMS now has 2 separate drug entries and can directly track usage from both K1 and T1 bottles. When you run the report for K1, it now shows every animal that received the drug and how much each animal received - this fulfills DEA reporting requirements.
Associated Topics:
medical-pharmacy inventory report
medical-drug usage report
Revised 5 March 2025
* Species360 Organizational name change added on 07/18/2016
It is the mission of Species360 to facilitate international collaboration in the collection and sharing of information on animals and their environments for zoos, aquariums and related organizations.
www.Species360.org – Global Information Serving Conservation





